Similar
A manual of practical medical electricity - the Röntgen rays and Finsen light (1902) (14597009828)
Zusammenfassung
Identifier: manualofpractica00turn (find matches)
Title: A manual of practical medical electricity : the Röntgen rays and Finsen light
Year: 1902 (1900s)
Authors: Turner, Dawson
Subjects: X-Rays Electrophysiology Electrosurgery Electric Stimulation Therapy Electrotherapeutics X-rays Electrophysiology Electrosurgery
Publisher: New York : William Wood & Company
Contributing Library: Francis A. Countway Library of Medicine
Digitizing Sponsor: Open Knowledge Commons and Harvard Medical School
Text Appearing Before Image:
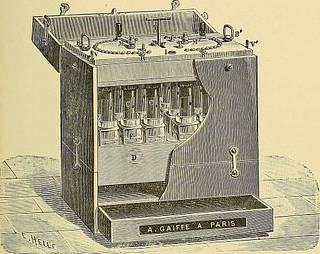
xes ; but owing to the solution creepingout and short-circuiting, it was not very durable. Ofthese faults others have also complained. It has beenpractically displaced in this country by some form ofLeclanche cell. The chloride of silver cell is naturally somewhat expen-sive, the small size costing 2s. lod. each ; but as the silveris not wasted, it can be cheaply recharged, and if itsdurability could be relied upon, it would be one of thebest portable medical cells. The Marie Davy or Persulphate of Mercury Cell. Of this there are also several forms. (a) The electrolyte is a solution of the persulphate ofmercury. (b) The positive element is of zinc. Forms of Primary Cells 43 (c) The negative element is of carbon. (d) The depolarizer is the persulphate of mercury.The E. M. F. is about i5 volts. The internal resistance is low. During action, the zinc decomposes the water, yieldinghydrogen, which, appearing at the carbon, displaces themercury, to form sulphuric acid and metallic mercury ;
Text Appearing After Image:
Fig. 20.—Persulphate of Mercury Cell Battery. the former attacks the zinc, while the latter collects at thebottom. In the old form, the cell resembled the Daniell, and hada porous pot, the sulphate of mercury taking the place ofthe sulphate of copper, and the carbon that of the copper.The porous pot is dispensed with in the later forms. Gaiffe uses a divided ebonite trough to form two smallcells. The carbon plates are placed at the bottom of the 44 A Manual of Practical Medical Electricity trough, and are smeared over with a paste of sulphate ofmercury ; the zincs form Hds which he over them. In Schanschieffs ceh there are two carbons to each zinc,and a strong solution of the basic sulphate is the excitant. In some of the later forms of sulphate of mercurybatteries, there is an arrangement for raising or loweringthe elements out of or into the solution, according to thestrength of current required, and, to insure portability,
Tags
Datum
Quelle
Copyright-info
























