Similar
An introductory course of lessons and exercises in chemistry (rewritten 1919) (1919) (14804518533)
Summary
Identifier: introductorycour00scho (find matches)
Title: An introductory course of lessons and exercises in chemistry (rewritten 1919)
Year: 1919 (1910s)
Authors: Schoch, Eugene Paul, 1871- (from old catalog)
Subjects: Chemistry
Publisher: (Austin) The Chemical laboratory of the University of Texas
Contributing Library: The Library of Congress
Digitizing Sponsor: The Library of Congress
Text Appearing Before Image:
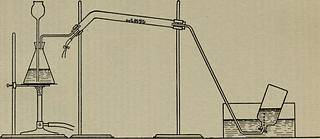
ustion tube heat the wateruntil a steady stream of steam is obtained. Temporarily discontinueheating the water, but warm up the tube until it is hot enough not tocondense steam; then connect the boiler to the tube, and start the steamcautiously through the tube, being careful to keep the tube hot enoughnot to condense steam, and also being careful to draw off through theextra tubing any water condensed in the lower part of the tube. Wheneverything is in proper working order, heat the tube strongly at the pointwhere the metal has been placed: when the proper temperature is reachedthe steam and the metal will react rapidly, and the hydrogen formedshould be collected in the bottle inverted over the end of the deliverytube. Ascertain if the gas collected is combustible. 3. The Reduction of Metallic Oxides:—The Reverse of the Pre-ceding Reaction. Xote.—The removal of oxygen from one of its compounds by theaction of a third substance—as illustrated in the reaction in this Chapter V 53
Text Appearing After Image:
54 Schoch: Inteoductoey Chemistey article—is called reduction. The latter term probably originatedfrom the metallurgists use of the term reduction to denote theextraction of metals from their ores: the ores reduced in fur-naces are generally oxides, and the chemical change consists inthe removal of the oxygen by a gas. It is the reverse of oxida-tion. Besides being used in their primary senses, the terms oxi-dation and reduction are also used in more extended senses. Thelatter will be shown later on. As might be expected, those metals which have a greater tend-ency than others to form compounds, exert also a greater resist-ance to being changed back to the metallic form—they are saidto be more difficult of reduction. The position of the metalsin the table given in Chapter III shows their relative ease or diffi-culty of reduction—the oxides of the metals in the lower part ofthe list are easily reduced, while the oxides of those in the upperpart are hard to reduce. It is on this a
Date
Source
Copyright info
















