Similar
Röntgen rays and electro-therapeutics - with chapters on radium and phototherapy (1910) (14571400530)
Summary
Identifier: rntgenrayselectr00kass (find matches)
Title: Röntgen rays and electro-therapeutics : with chapters on radium and phototherapy
Year: 1910 (1910s)
Authors: Kassabian, Mihran Krikor, 1870-1910
Subjects: Electrotherapeutics X-rays Phototherapy Radiology Radiotherapy
Publisher: Philadelphia & London : J.B. Lippincott Company
Contributing Library: Francis A. Countway Library of Medicine
Digitizing Sponsor: Open Knowledge Commons and Harvard Medical School
Text Appearing Before Image:
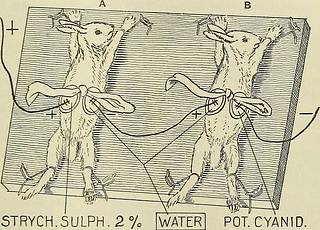
nide anion, and showing that the result wasnot due to ordinary absorption. Again, Leduc has shown that whenpotassium permanganate is used as the cathode, the anion (the perman-ganic acid radical) penetrates the skin and colors it; per contra, no suchcoloration takes place when the permanganate solution is used as ananode. The glandular orifices are the avenues by which the ions andthe electric current penetrate the body. IONIC THERAPY. 51 Sensations Eesulting from Integumentary Penetration of Ions. The introdnctiou into the skiu of each varietj^ of ion is accompaniedby a special sensation. The anions CI, Br, and I cause merely a slightsensation of heat, the cathions K and )Na provoke painful burningsensations. Ba, Ca, Fe, S, Zn, and Mg are all i^ainful. The ions of thehea\y metals coagulate albumen and are destructive to the integument.Au, Pb, and Ag are quite painless. It is worthy of note that the inorganic salts dissociate more easilythan the organic, and the same is true of bases.
Text Appearing After Image:
STRYCH.SULPH.2 7. Cathion. Fig. 33.—Penetration of ions through the intesuraent. POT. CYANID. Anion (Leducs Experiment.) C. Therapeutic Action as a Eesult of Dissociation. Sodium hydrate has a peculiar action on the tissues, causing corro-sion; as the Xa ion is present in many solutions that are non-corrosivein character, it is fair to infer that the hydroxyl ion is corrosive in itseffects. Again, alcohol contains an hydroxyl, and yet it has no suchcorrosive action; this corresponds with the fact that alcohol is not anelectrolyte, hence there is no liberation of its hydroxyl ion. As a general rule one ion is so powerful that, therapeutically, theother may be ignored. Thus, in morphine sulphate, the alkaloidal cathionis so active that the sulphate ion may be ignored, and the sulphate has,therefore, ^he same effect as the hydroclilorate of morphine An interesting study is the consideration of ions in the therapeuticemployment of the bromides. These have a depressing action on thecentr
Tags
Date
Source
Copyright info
























