Similar
Elements of modern chemistry (1887) (14768351544)
Summary
Identifier: elementsofmode00wurt (find matches)
Title: Elements of modern chemistry
Year: 1887 (1880s)
Authors: Wurtz, Charles Adolphe, 1817-1884 Greene, William H. (William Houston), 1853-1918, ed. and tr
Subjects: Chemistry
Publisher: Philadelphia, J.B. Lippincott company
Contributing Library: The Library of Congress
Digitizing Sponsor: The Library of Congress
Text Appearing Before Image:
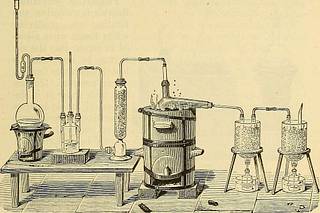
- C=^ -f CI* = SiCl* + 2C0 Preparation.—Precipitated silica, lamp-black, and oil areintimately mixed into a stifi paste. This paste is made intolittle balls, which are put into a crucible, the cover of which isthen luted on, and the whole is heated to redness in a furnace.When cool, the balls are introduced into a porcelain tube or aclay retort (Fig. 74), which is then heated to bright redness,while a current of carefully-dried chlorine is passed through.The silicon chloride and the carbon monoxide formed are SILICON FLUORIDE. 197 passed through two U tubes surrounded by a mixture of iceand salt. The siHcon chloride is thus condensed. Properties.—Silicon chloride is a volatile, colorless liquid,of an irritating odor. It fumes in the air. Its density is 1.52,and it boils at 59°. It is instantly decomposed by water, silicic and hydrochloricacids being formed. A part of the silicic acid is precipitated
Text Appearing After Image:
Fig. 74. in the form of a jelly, while another part remains in solution.The latter is perhaps a hydrate corresponding to the chloride.SiCl* -I- 4.W0 = 4HC1 + Si(OH)* There exists a tetrabromide of silicon, SiBr*, and a tetra-iodide, SiP, both corresponding to the chloride which has justbeen described. Friedel has recently discovered an iodide, Si^P, remarkableas belonging to an entirely new series. SILICOM FLUORIDE. SiFl* Density compared to air 3.6 Density compared to hydrogen 52. Preparation.—An intimate mixture of silicious sand and17* 198 ELEMENTS OF MODERN CHEMISTRY. finely-powdered calcium fluoride, or fluor spar, is introducedinto a glass flask (Fig. 75), and a sufficient quantity of sul-phuric acid is added to reduce the whole to a creamy consistence.A gentle heat is applied, and the gas disengaged may be col-lected over mercury.2CaFP + 2H^S0* + SiO^ = 2CaS0* + SiFl* + 2W0 Calcium fluoride. Silicic oxide. Calcium sulphate. Properties.—S i 1 i c o nfluoride is a colorless,
Tags
Date
Source
Copyright info





![[Electrical Tools/Mobile Exhibition] [Electrical Tools/Mobile Exhibition]](https://cache.getarchive.net/Prod/thumb/cdn10/L3Bob3RvLzE5NjcvMTIvMzEvZWxlY3RyaWNhbC10b29sc21vYmlsZS1leGhpYml0aW9uLTM4MzQzMS02NDAuanBn/40/32/jpg)









